A Game-Changer in Medicine: Understanding Lipid Nanoparticle Formulation
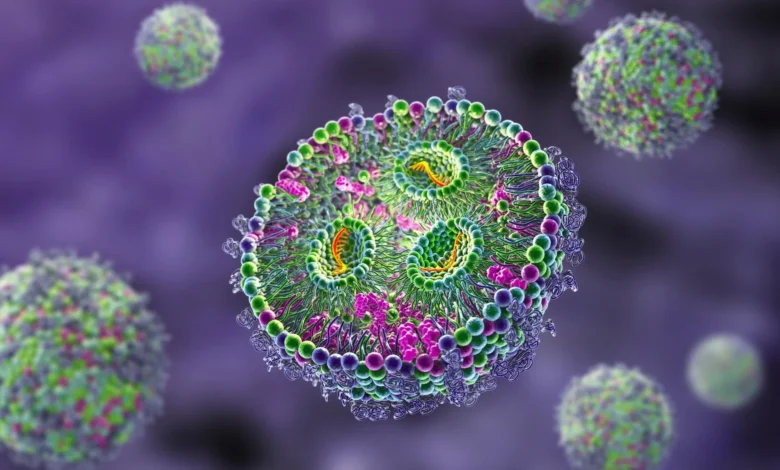
Table of Contents:
- The Pioneering Role of Lipid Nanoparticles in mRNA Therapeutics
- Breaking Down the Science of LNPs
- Benefits and Challenges of LNP-Formulated Therapeutics
- Real-World Impact: LNP-Formulated Medicines in Action
- Quality and Safety: Regulation of LNP Technologies
- Understanding the Broader Implications of LNP Technology
Key Takeaways
- Lipid nanoparticles (LNPs) are critical in delivering mRNA-based therapeutics more effectively.
- LNPs have played a pivotal role in developing COVID-19 vaccines, showcasing their importance in modern medicine.
- The future of LNP research is promising, with potential breakthroughs in personalized medicine and beyond.
The Pioneering Role of Lipid Nanoparticles in mRNA Therapeutics
A silent revolution within the medical field hinges on a remarkable technology known as lipid nanoparticle formulation. These minute particles are the linchpin in the delivery method of mRNA-based therapeutics, offering a means to prompt our bodies to produce therapeutic proteins. Through this innovative method, gene therapies have seen a renaissance, and vaccines have been produced at record speeds in response to the COVID-19 pandemic, a testament to the transformative power of LNPs in the healthcare sector.
The value of lipid nanoparticles goes beyond their current use. They are seen as a bridge to a future where medicine is more responsive, adaptive, and personalized. With their ability to securely carry genetic instructions into human cells, LNPs represent a step forward in medicine and a leap into new realms of possibility. This groundbreaking technology contributes to a fundamental shift in how we approach diseases, enabling healthcare professionals to treat the previously untreatable.
Breaking Down the Science of LNPs
Lipid nanoparticle formulation is expertly designed to act as trojan horses, cleverly engineered to bypass the body’s defense mechanisms and deliver nucleic acids directly into cells for therapeutic purposes. The formulations consist of a well-selected blend of lipids. Different lipids play different roles in the mixture, such as providing stability and rigidity, facilitating endosomal escape, and ensuring the biodegradability of the nanoparticles. The creation of these particles involves a process called ‘nanoprecipitation.’ This process entails carefully mixing an organic solvent containing the lipids and the mRNA with an aqueous solution. It leads to the spontaneous assembly of lipid nanoparticles. The key to success is precisely controlling the chemistry involved in this process to create uniformly stable LNPs consistently. Scientists are continuously refining this process to keep pace with the latest advancements in the scientific field.
Benefits and Challenges of LNP-Formulated Therapeutics
Lipid nanoparticles offer a suite of benefits, making them an attractive drug delivery choice. For one, their ability to precisely target specific cells or tissues minimizes the side effects that typically accompany systemic drug administration. This targeting is critical in reducing adverse events and improving patient tolerability. Moreover, LNPs can be engineered to release their payload at controlled rates, offering a sustained therapeutic effect that can be optimized for different treatment regimens.
Yet, the journey from concept to clinic is fraught with hurdles. One such challenge is maintaining the integrity of the lipid nanoparticles over time. Stability is a prerequisite for the efficacy and scalability of LNP production, which is crucial as we move towards bringing these treatments to market. To address these issues, research is delving into new lipid formulations and manufacturing processes that can withstand the rigors of scale without compromising quality.
Furthermore, there is an ongoing quest to understand and mitigate the potential immune responses elicited by lipid nanoparticles. This area is as complex as critical for the success of LNP-based therapies. Navigating these challenges reveals the tenacity and innovative spirit that permeate the LNP research and development field.
Real-World Impact: LNP-Formulated Medicines in Action
The accurate measure of any medical innovation lies in its real-world implications, and lipid nanoparticles have proven their mettle. The most salient example of their impact is the rapid mobilization of LNP-based vaccines in response to the COVID-19 pandemic. These vaccines have shifted the trajectory of the pandemic, demonstrating not only the potential but also the real-world effectiveness of lipid nanoparticle technologies in protecting millions of lives across the globe.
Aside from their heralded success in infectious diseases, lipid nanoparticles are also carving out a niche in the fight against more insidious foes, such as cancer and rare genetic disorders. For instance, in the case of cancer, where precision and targeted therapies can make the difference between life and death, LNPs provide a delivery method that ensures the therapeutic agent reaches only the tumor cells, thus sparing the rest of the body’s healthy cells.
The versatility of LNPs is a beacon of hope for the future of medicine. It’s a foundation upon which more efficient, effective, and patient-friendly therapies can be developed. By surmounting the biological barriers that have historically made treatment so challenging, LNPs herald a new era of medical treatments and increased quality of life for people with various conditions.
Quality and Safety: Regulation of LNP Technologies
The innovation of lipid nanoparticle technologies brings with it a responsibility to ensure they are as safe as they are effective. Regulatory bodies have the critical task of overseeing these technologies and adapting existing frameworks to accommodate their unique challenges. This oversight ensures that the products reaching patients are held to the highest quality control standards.
Contemporary LNP production operates under stringent guidelines that govern everything from the sourcing of raw materials to the scale-up of manufacturing processes. These guidelines are in place to protect patient safety, ensuring that every batch of nanoparticles exhibits a consistent therapeutic profile, with rigorous testing protocols to screen for potency, purity, and stability. It’s a complex exercise in balance: encouraging innovative treatments while safeguarding the public health interest.
Understanding the Broader Implications of LNP Technology
The scope of lipid nanoparticle technology extends well beyond the confines of research laboratories and hospital wards. With the ability to facilitate revolutionary treatments, LNPs carry the potential to reshape health policies and access on a global scale. They serve as harbingers of a future healthcare paradigm marked by rapid responses to emerging health threats and the ability to tailor treatments to individual patient needs.
However, the dazzling promise of LNPs is clear of the genuine challenges associated with their dissemination. Equity in access to these life-saving technologies is a complex issue encompassing economics, logistics, and moral imperatives. As we stand on the cusp of a new epoch in medicine, it is the collective responsibility of the scientific community, healthcare providers, policymakers, and society to ensure that advancements in LNP technology are leveraged for the benefit of all humankind, not just a privileged few.
Moreover, cultivating a well-informed public is critical for the acceptance and success of LNP-based treatments. Knowledge empowers individuals to make informed decisions about their health and promotes a supportive environment where medical advancements can be implemented effectively. As LNPs become an ever-more prominent feature in the therapeutic landscape, open dialogue, and educational outreach are paramount to ensuring that the public remains engaged and that the full potential of this groundbreaking technology is realized.





